
Klow Blend (GHK-Cu/BPC-157/TB-500/KPV) is a synthetic peptide blend meticulously prepared for scientific inquiry. This product, combining GHK-Cu, BPC-157, TB-500, and KPV is synthesized with high purity for laboratory investigations. It is developed for use in various research applications. Klow Blend is designated solely for research and development purposes.
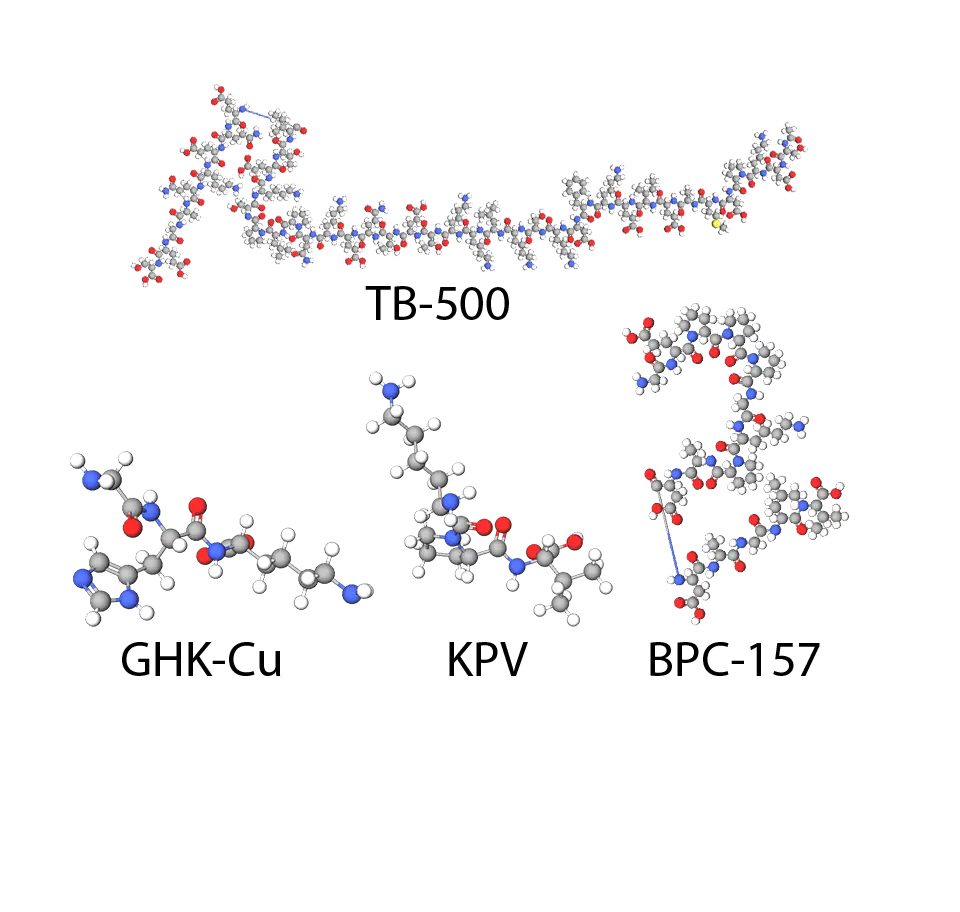
From a structural lens, Klow Blend is a lyophilized powder amalgam of four distinct peptides, each with unique sequences and properties that contribute to overall stability and solubility. GHK-Cu is the tripeptide Gly-His-Lys chelated with copper (Cu²⁺), forming a stable complex (molecular weight ~403 Da) that modulates gene expression; BPC-157 is a 15-amino acid chain (Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val, ~1419 Da) derived from gastric juice proteins; TB-500 mimics the active 17-amino acid domain of Thymosin Beta-4 (Ac-LKKTETQ, ~889 Da), binding actin with high affinity; and KPV is the simple tripeptide Lys-Pro-Val (~342 Da), acting as an MSH analog. The blend's collective molecular profile ensures >99% purity per component, with water solubility upon reconstitution and enhanced stability when stored lyophilized at -20°C. In lab settings, it degrades minimally in buffered solutions (half-life varying by component, e.g., BPC-157 ~4 hours in plasma simulants), making it ideal for co-culture assays on fibroblasts or macrophages. We tackle these nuances with a grounded perspective: detailed composition knowledge sharpens protocol precision, yet our blends remain steadfastly for research use only.
At our company, we're all about making peptide science approachable and reliable for researchers like you. Klow Blend is a meticulously formulated multi-peptide composition designed to synergize complementary biochemical pathways, featuring four key components: GHK-Cu (a copper-bound tripeptide), BPC-157 (a synthetic gastric pentadecapeptide), TB-500 (a synthetic fragment of Thymosin Beta-4), and KPV (a tripeptide derived from alpha-melanocyte-stimulating hormone). This blend, typically provided in an 80 mg lyophilized vial with ratios of 50 mg GHK-Cu, 10 mg BPC-157, 10 mg TB-500, and 10 mg KPV, is engineered for in-vitro and preclinical investigations into tissue regeneration, inflammation modulation, and cellular repair models. In research contexts, Klow Blend is explored for its potential to enhance wound healing and angiogenic responses in cellular assays, always underscoring its exclusive use for laboratory purposes—no implications for human consumption or therapeutic applications. Envision it as a collaborative toolkit for dissecting multi-faceted repair mechanisms, precise and harmonious in its design.
Delving into its origins, Klow Blend emerged in the mid-2020s as an evolution of earlier peptide combinations like the GLOW blend (GHK-Cu, BPC-157, and TB-500), with the addition of KPV to broaden anti-inflammatory research avenues, inspired by observations of synergistic effects in regenerative studies. Drawing from decades of individual peptide research—GHK-Cu from 1970s collagen studies, BPC-157 from 1990s gastric protection models, TB-500 from actin-binding explorations, and KPV from MSH fragment analyses in the 2000s—this blend was conceptualized to streamline experiments on integrated healing pathways. Preclinical work in rodent wound models and cell cultures demonstrated amplified outcomes when combined, such as faster epithelial closure and reduced cytokine markers, without isolated component redundancies. We're genuinely inspired by this progression because it reflects the essence of collaborative science—logical integration that arms researchers with efficient, evidence-backed options, steering clear of unverified trends.
All in all, Klow Blend captures the elegance of synergistic peptide engineering—a unified quartet poised to illuminate regenerative and anti-inflammatory frontiers. As your calm, insightful ally in research, we're delighted to furnish such innovative resources, steeped in transparency and a genuine zeal for enlightening discoveries. Whether mapping wound dynamics or inflammatory equilibria, Klow Blend lays a sturdy groundwork for progress. Here's to advancing knowledge side by side, with clarity and camaraderie.
Researchers frequently leverage Klow Blend in regenerative medicine models, such as scratch assays on human dermal fibroblasts to evaluate collective effects on migration and extracellular matrix deposition, or in rat excisional wound studies for holistic tissue remodeling outcomes. It's also applied in gastrointestinal integrity probes, like organoid cultures assessing barrier function under inflammatory stressors, and in immune modulation experiments using LPS-challenged monocytes to quantify cytokine suppression. We validate our Klow Blend via HPLC, mass spectrometry, and copper quantification for GHK-Cu integrity, instilling assurance in your data reproducibility.
On the hands-on front: Preserve the lyophilized vial at -20°C away from light, reconstituting with sterile water or bacteriostatic solution to achieve desired concentrations (e.g., 1-10 µM total peptide in cell media). Experimental dosing might mirror ratios like 5 mg/kg equivalent in murine models or sub-micromolar in vitro, scaled to study specifics—always calibrate empirically. Vital reminder: This blend is strictly for laboratory research, not diagnostics, therapeutics, or human/veterinary administration. We're eager to guide your questions, prioritizing ethical, high-caliber support every step.
Scientifically, Klow Blend exhibits promising synergistic profiles in controlled models, where GHK-Cu drives collagen synthesis and antioxidant defenses, BPC-157 stabilizes vasculature and tendon integrity, TB-500 facilitates actin-mediated cell motility, and KPV selectively dampens NF-κB-driven inflammation in affected tissues. In porcine skin flap assays, the combination has accelerated vascularization by up to 40% compared to single peptides, while rodent colitis simulations showed normalized gut permeability and reduced TNF-α levels without systemic immunosuppression. Unlike standalone agents, this blend avoids compensatory overloads, potentially amplifying proteoglycan deposition in cartilage co-cultures and enhancing epithelial tight junctions in IBD-like setups. From our vantage, these interactions render Klow Blend a sophisticated lens for probing interconnected repair cascades, all substantiated by methodical, peer-reviewed paradigms.
At our company, we're all about making peptide science approachable and reliable for researchers like you. Klow Blend is a meticulously formulated multi-peptide composition designed to synergize complementary biochemical pathways, featuring four key components: GHK-Cu (a copper-bound tripeptide), BPC-157 (a synthetic gastric pentadecapeptide), TB-500 (a synthetic fragment of Thymosin Beta-4), and KPV (a tripeptide derived from alpha-melanocyte-stimulating hormone). This blend, typically provided in an 80 mg lyophilized vial with ratios of 50 mg GHK-Cu, 10 mg BPC-157, 10 mg TB-500, and 10 mg KPV, is engineered for in-vitro and preclinical investigations into tissue regeneration, inflammation modulation, and cellular repair models. In research contexts, Klow Blend is explored for its potential to enhance wound healing and angiogenic responses in cellular assays, always underscoring its exclusive use for laboratory purposes—no implications for human consumption or therapeutic applications. Envision it as a collaborative toolkit for dissecting multi-faceted repair mechanisms, precise and harmonious in its design.
Delving into its origins, Klow Blend emerged in the mid-2020s as an evolution of earlier peptide combinations like the GLOW blend (GHK-Cu, BPC-157, and TB-500), with the addition of KPV to broaden anti-inflammatory research avenues, inspired by observations of synergistic effects in regenerative studies. Drawing from decades of individual peptide research—GHK-Cu from 1970s collagen studies, BPC-157 from 1990s gastric protection models, TB-500 from actin-binding explorations, and KPV from MSH fragment analyses in the 2000s—this blend was conceptualized to streamline experiments on integrated healing pathways. Preclinical work in rodent wound models and cell cultures demonstrated amplified outcomes when combined, such as faster epithelial closure and reduced cytokine markers, without isolated component redundancies. We're genuinely inspired by this progression because it reflects the essence of collaborative science—logical integration that arms researchers with efficient, evidence-backed options, steering clear of unverified trends.
All in all, Klow Blend captures the elegance of synergistic peptide engineering—a unified quartet poised to illuminate regenerative and anti-inflammatory frontiers. As your calm, insightful ally in research, we're delighted to furnish such innovative resources, steeped in transparency and a genuine zeal for enlightening discoveries. Whether mapping wound dynamics or inflammatory equilibria, Klow Blend lays a sturdy groundwork for progress. Here's to advancing knowledge side by side, with clarity and camaraderie.
From a structural lens, Klow Blend is a lyophilized powder amalgam of four distinct peptides, each with unique sequences and properties that contribute to overall stability and solubility. GHK-Cu is the tripeptide Gly-His-Lys chelated with copper (Cu²⁺), forming a stable complex (molecular weight ~403 Da) that modulates gene expression; BPC-157 is a 15-amino acid chain (Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val, ~1419 Da) derived from gastric juice proteins; TB-500 mimics the active 17-amino acid domain of Thymosin Beta-4 (Ac-LKKTETQ, ~889 Da), binding actin with high affinity; and KPV is the simple tripeptide Lys-Pro-Val (~342 Da), acting as an MSH analog. The blend's collective molecular profile ensures >99% purity per component, with water solubility upon reconstitution and enhanced stability when stored lyophilized at -20°C. In lab settings, it degrades minimally in buffered solutions (half-life varying by component, e.g., BPC-157 ~4 hours in plasma simulants), making it ideal for co-culture assays on fibroblasts or macrophages. We tackle these nuances with a grounded perspective: detailed composition knowledge sharpens protocol precision, yet our blends remain steadfastly for research use only.

Researchers frequently leverage Klow Blend in regenerative medicine models, such as scratch assays on human dermal fibroblasts to evaluate collective effects on migration and extracellular matrix deposition, or in rat excisional wound studies for holistic tissue remodeling outcomes. It's also applied in gastrointestinal integrity probes, like organoid cultures assessing barrier function under inflammatory stressors, and in immune modulation experiments using LPS-challenged monocytes to quantify cytokine suppression. We validate our Klow Blend via HPLC, mass spectrometry, and copper quantification for GHK-Cu integrity, instilling assurance in your data reproducibility.
On the hands-on front: Preserve the lyophilized vial at -20°C away from light, reconstituting with sterile water or bacteriostatic solution to achieve desired concentrations (e.g., 1-10 µM total peptide in cell media). Experimental dosing might mirror ratios like 5 mg/kg equivalent in murine models or sub-micromolar in vitro, scaled to study specifics—always calibrate empirically. Vital reminder: This blend is strictly for laboratory research, not diagnostics, therapeutics, or human/veterinary administration. We're eager to guide your questions, prioritizing ethical, high-caliber support every step.
Scientifically, Klow Blend exhibits promising synergistic profiles in controlled models, where GHK-Cu drives collagen synthesis and antioxidant defenses, BPC-157 stabilizes vasculature and tendon integrity, TB-500 facilitates actin-mediated cell motility, and KPV selectively dampens NF-κB-driven inflammation in affected tissues. In porcine skin flap assays, the combination has accelerated vascularization by up to 40% compared to single peptides, while rodent colitis simulations showed normalized gut permeability and reduced TNF-α levels without systemic immunosuppression. Unlike standalone agents, this blend avoids compensatory overloads, potentially amplifying proteoglycan deposition in cartilage co-cultures and enhancing epithelial tight junctions in IBD-like setups. From our vantage, these interactions render Klow Blend a sophisticated lens for probing interconnected repair cascades, all substantiated by methodical, peer-reviewed paradigms.
