
Melanotan II, or MT-2, is a synthetic peptide meticulously prepared for scientific inquiry. This product is synthesized with high purity for laboratory investigations. It is developed for use in various research applications. Melanotan II is designated solely for research and development purposes.
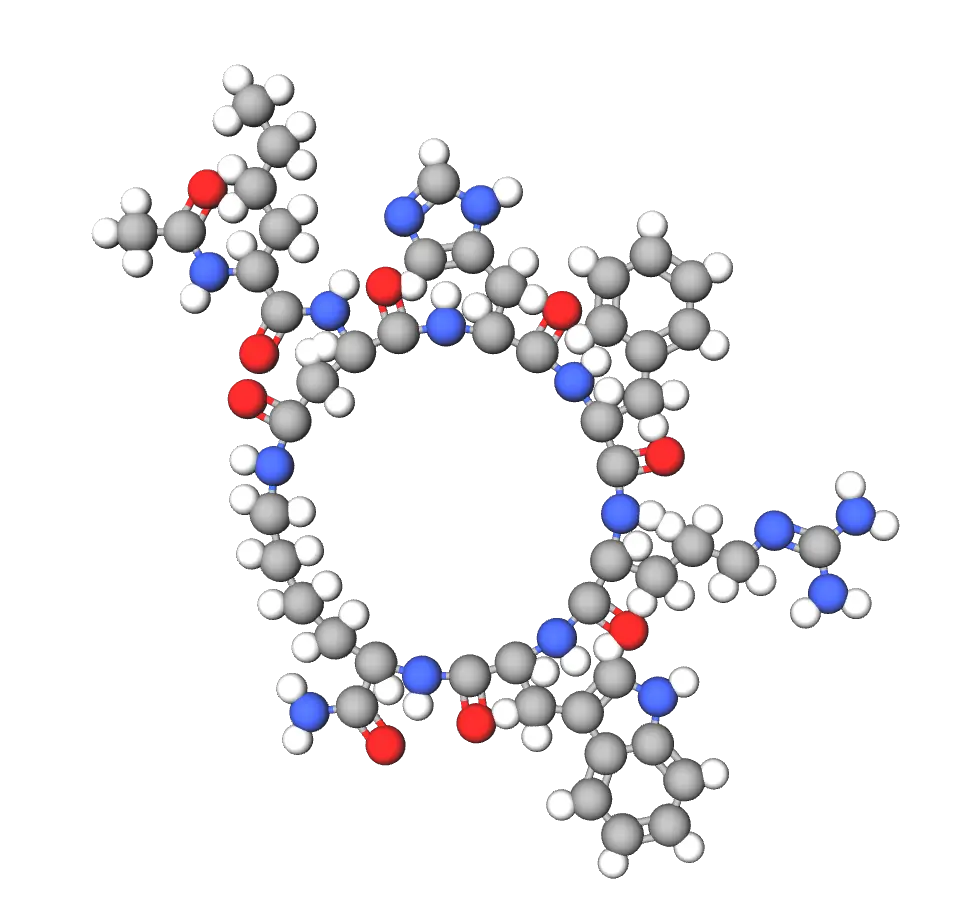
Melanotan II's structure is a cyclic analog with the sequence Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH2, corresponding to Ac-Nle4-Asp5-His6-D-Phe7-Arg8-Trp9-Lys10-α-MSH4-10-NH2, featuring a lactam bridge between positions 5 and 10 for increased stability and potency. Its molecular formula is C50H69N15O9, with a molecular weight of approximately 1024.18 g/mol, and it incorporates non-natural amino acids like norleucine (Nle) and D-phenylalanine (D-Phe) to enhance resistance to enzymatic breakdown and receptor binding affinity. This cyclic design makes it about 1,000 times more potent than α-MSH in melanotropic assays, allowing for effective use in low concentrations during experiments.
In practical lab terms, Melanotan II is supplied as a lyophilized powder with purity levels typically over 98%, confirmed by HPLC and mass spectrometry. It demonstrates good solubility in aqueous buffers, but its cyclic nature may require careful pH control to maintain conformational integrity. Our transparent quality processes ensure each batch is tested for consistency, so you can rely on it for precise, reproducible results in your studies.
Melanotan II is a synthetic cyclic heptapeptide analog of alpha-melanocyte-stimulating hormone (α-MSH), engineered to activate melanocortin receptors and promote melanogenesis in research models. This potent peptide, with its lactam-bridged structure, exhibits enhanced affinity for multiple melanocortin receptor subtypes compared to native α-MSH, making it a valuable tool for in-vitro investigations into pigmentation, photoprotection, and related physiological pathways. As with all our peptides, Melanotan II is intended strictly for research purposes and in-vitro use only—not for human consumption or any diagnostic or therapeutic applications.
Melanotan II functions as a non-selective agonist at melanocortin receptors, including MC1R, MC3R, MC4R, and MC5R, with varying potencies across subtypes. Through activation of MC1R on melanocytes, it elevates cAMP levels, leading to upregulation of tyrosinase and other enzymes involved in eumelanin synthesis, thereby enhancing pigmentation and photoprotective responses. Engagement with MC4R may influence central pathways related to appetite suppression and sexual arousal, potentially via modulation of neuronal signaling, while MC3R and MC5R contribute to broader metabolic and inflammatory effects.
In cellular models, this agonism can induce effects such as reduced food intake through hypothalamic MC4R activation, with tolerance developing over time, suggesting adaptation to altered energy set points. Additional observations include anti-inflammatory actions via NF-κB inhibition and potential modulation of pain pathways, where it may exacerbate allodynia in neuropathic models. We enjoy simplifying these concepts—Melanotan II acts like a multifaceted switch for melanocortin systems, enabling logical exploration of how receptor interactions drive diverse biological outcomes in isolated systems.
Ultimately, Melanotan II represents a superpotent cyclic analog of α-MSH, providing researchers with a versatile probe for unraveling melanocortin receptor dynamics across pigmentation, metabolism, and beyond. Its selective yet broad actions inspire our passion for demystifying peptide science through quality resources and open dialogue. Strictly for in-vitro research, Melanotan II reflects our humble confidence in delivering tools that foster meaningful discoveries with integrity. Let's build on this together, one well-reasoned experiment at a time.
Melanotan II's broad receptor profile positions it as a key asset for in-vitro and ex-vivo research in dermatology, endocrinology, and neuroscience. In melanocyte cultures, it has been employed to investigate melanogenesis pathways, demonstrating increased eumelanin production and reduced UV-induced damage, which supports studies on photoprotection and skin cancer models. Research has also explored its effects on melanoma progression, with in-vivo models showing suppression of tumor growth, highlighting its potential in oncology assays.
In metabolic studies, Melanotan II aids in probing appetite regulation via MC4R, where it transiently suppresses feeding in cellular and tissue models, offering insights into obesity mechanisms and body fat homeostasis. Neurological applications include examining its role in sexual dysfunction through MC4R-mediated arousal pathways, as well as pain modulation in neuropathic models, where antagonists might reveal therapeutic angles. We're committed to educational support for these uses—consider it for comparative receptor-binding assays or combined with UV stressors to uncover synergies—and our team can provide tips on optimizing protocols for your specific questions.
To keep Melanotan II at peak condition for your experiments, store the lyophilized powder at -20°C or below in a desiccated, light-protected environment to preserve its cyclic structure and prevent degradation. For reconstitution, use sterile water or a neutral buffer to reach concentrations of 1-2 mg/mL, gently mixing to avoid disrupting the lactam bridge—vigorous shaking could lead to conformational changes.
Prepared solutions are best used fresh, but can be aliquoted and frozen, with minimal freeze-thaw cycles recommended. Our stability testing ensures it withstands these practices, but always verify pH and clarity before use. If you need advice on assay compatibility or adjustments, we're here with calm, rational suggestions based on solid science.
Melanotan II is a synthetic cyclic heptapeptide analog of alpha-melanocyte-stimulating hormone (α-MSH), engineered to activate melanocortin receptors and promote melanogenesis in research models. This potent peptide, with its lactam-bridged structure, exhibits enhanced affinity for multiple melanocortin receptor subtypes compared to native α-MSH, making it a valuable tool for in-vitro investigations into pigmentation, photoprotection, and related physiological pathways. As with all our peptides, Melanotan II is intended strictly for research purposes and in-vitro use only—not for human consumption or any diagnostic or therapeutic applications.
Melanotan II functions as a non-selective agonist at melanocortin receptors, including MC1R, MC3R, MC4R, and MC5R, with varying potencies across subtypes. Through activation of MC1R on melanocytes, it elevates cAMP levels, leading to upregulation of tyrosinase and other enzymes involved in eumelanin synthesis, thereby enhancing pigmentation and photoprotective responses. Engagement with MC4R may influence central pathways related to appetite suppression and sexual arousal, potentially via modulation of neuronal signaling, while MC3R and MC5R contribute to broader metabolic and inflammatory effects.
In cellular models, this agonism can induce effects such as reduced food intake through hypothalamic MC4R activation, with tolerance developing over time, suggesting adaptation to altered energy set points. Additional observations include anti-inflammatory actions via NF-κB inhibition and potential modulation of pain pathways, where it may exacerbate allodynia in neuropathic models. We enjoy simplifying these concepts—Melanotan II acts like a multifaceted switch for melanocortin systems, enabling logical exploration of how receptor interactions drive diverse biological outcomes in isolated systems.
Ultimately, Melanotan II represents a superpotent cyclic analog of α-MSH, providing researchers with a versatile probe for unraveling melanocortin receptor dynamics across pigmentation, metabolism, and beyond. Its selective yet broad actions inspire our passion for demystifying peptide science through quality resources and open dialogue. Strictly for in-vitro research, Melanotan II reflects our humble confidence in delivering tools that foster meaningful discoveries with integrity. Let's build on this together, one well-reasoned experiment at a time.
Melanotan II's structure is a cyclic analog with the sequence Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH2, corresponding to Ac-Nle4-Asp5-His6-D-Phe7-Arg8-Trp9-Lys10-α-MSH4-10-NH2, featuring a lactam bridge between positions 5 and 10 for increased stability and potency. Its molecular formula is C50H69N15O9, with a molecular weight of approximately 1024.18 g/mol, and it incorporates non-natural amino acids like norleucine (Nle) and D-phenylalanine (D-Phe) to enhance resistance to enzymatic breakdown and receptor binding affinity. This cyclic design makes it about 1,000 times more potent than α-MSH in melanotropic assays, allowing for effective use in low concentrations during experiments.
In practical lab terms, Melanotan II is supplied as a lyophilized powder with purity levels typically over 98%, confirmed by HPLC and mass spectrometry. It demonstrates good solubility in aqueous buffers, but its cyclic nature may require careful pH control to maintain conformational integrity. Our transparent quality processes ensure each batch is tested for consistency, so you can rely on it for precise, reproducible results in your studies.

Melanotan II's broad receptor profile positions it as a key asset for in-vitro and ex-vivo research in dermatology, endocrinology, and neuroscience. In melanocyte cultures, it has been employed to investigate melanogenesis pathways, demonstrating increased eumelanin production and reduced UV-induced damage, which supports studies on photoprotection and skin cancer models. Research has also explored its effects on melanoma progression, with in-vivo models showing suppression of tumor growth, highlighting its potential in oncology assays.
In metabolic studies, Melanotan II aids in probing appetite regulation via MC4R, where it transiently suppresses feeding in cellular and tissue models, offering insights into obesity mechanisms and body fat homeostasis. Neurological applications include examining its role in sexual dysfunction through MC4R-mediated arousal pathways, as well as pain modulation in neuropathic models, where antagonists might reveal therapeutic angles. We're committed to educational support for these uses—consider it for comparative receptor-binding assays or combined with UV stressors to uncover synergies—and our team can provide tips on optimizing protocols for your specific questions.
To keep Melanotan II at peak condition for your experiments, store the lyophilized powder at -20°C or below in a desiccated, light-protected environment to preserve its cyclic structure and prevent degradation. For reconstitution, use sterile water or a neutral buffer to reach concentrations of 1-2 mg/mL, gently mixing to avoid disrupting the lactam bridge—vigorous shaking could lead to conformational changes.
Prepared solutions are best used fresh, but can be aliquoted and frozen, with minimal freeze-thaw cycles recommended. Our stability testing ensures it withstands these practices, but always verify pH and clarity before use. If you need advice on assay compatibility or adjustments, we're here with calm, rational suggestions based on solid science.
